News Story
Computational framework automatically optimizes the shape of tissue engineered vascular grafts
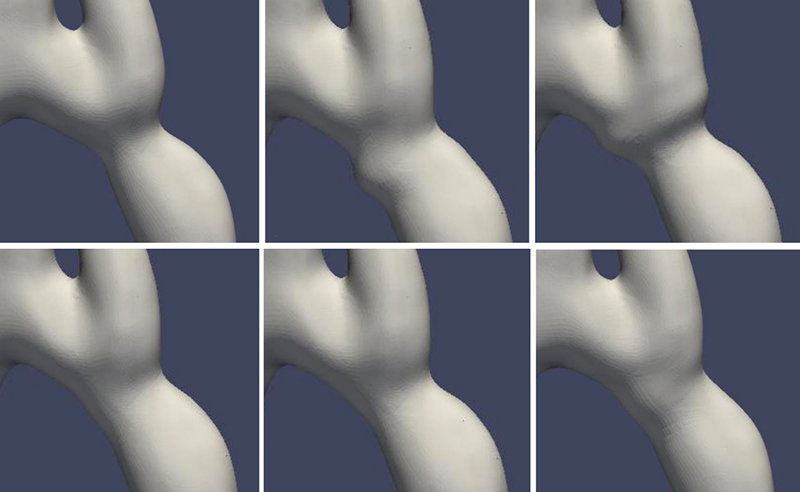
Illustrations of various shapes that can be achieved through the shape deformation algorithm with randomly sampled design parameters. (Fig. 2 from the paper)
Congenital heart disease (CHD) affects nearly 40,000 infants in the U.S. every year and is among the leading causes of newborn death. Approximately 25% of babies born with CHD require surgical or other potentially lifesaving treatments.
In particular, aortic arch anomalies such as aortic coarctation and hypoplasia often require early surgical intervention. In cases of significant associated arch hypoplasia, an aortic arch reconstruction may be required. Current techniques can result in distortion of the aortic arch shape, and are limited by available synthetic materials that do not grow with the child and require revision or replacement. Biocompatible materials, including autografts and allografts, work well, although they have a limited availability and can be prone to dilation over time.
Tissue engineered vascular grafts (TEVGs) offer a promising strategy for overcoming such complications. Using an FDA-approved biodegradable scaffold, the patient’s own cells can proliferate and provide physiologic functionality and growth over time.
Electrospun nanofibers can be deposited on a 3D-printed stainless steel mandrel to create TEVGs with custom shapes. The feasibility of customizing the shapes of TEVGs opens the door to designing patient-specific grafts that can achieve optimal outcomes during aorta repair. Surgical intuition can be combined with computational predictions of hemodynamics to achieve an optimized, durable, patient-specific design for aortic arch repair.
A new paper, Automatic Shape Optimization of Patient-Specific Tissue Engineered Vascular Grafts for Aortic Coarctation, develops a computational framework for automatically designing optimal shapes of patient-specific TEVGs for aorta surgery. It was written by Mechanical Engineering Postdoctoral Researcher Xiaolong Liu, Mechanical Engineering Ph.D. students Seda Aslan and Rachel Hess; ISR-affiliated Assistant Professor Mark Fuge (ME) and Assistant Professor Axel Krieger (ME); Paige Mass, Laura Olivieri, and Yue-Hin Loke of the Sheikh Zayed Institute for Pediatric Surgical Innovation, Children’s National Hospital, Washington, D.C.; and Narutoshi Hibino, Department of Cardiac Surgery, University of Chicago Medicine.
The work was presented at the 42nd Annual International Conference of the IEEE Engineering in Medicine and Biology Society.
The researchers demonstrate a computational framework for automatically optimizing the shape of patient-specific tissue engineered vascular grafts and apply this computational framework to a case of aortic coarctation for proof-of-concept. By controlling a set of design parameters to deform a cylindrical lattice applied on a patient’s native aorta model, various geometric shapes are generated for computing values of the objective function by computational fluid dynamics (CFD) simulation.
Instead of using commercial CFD software, the researchers employ an open-source CFD solver (OpenFOAM) which is seamlessly integrated into the computational framework. Based on the observation data from CFD simulation, the hemodynamic surrogate model for each patient’s aorta is trained by using Gaussian process regression. The globally optimal design parameters can be found by running a multi-start conjugate gradient optimization on the surrogate model for computing the final geometric shapes of the TEVGs.
The researchers demonstrate an automatic optimization pipeline for designing TEVGs and an effective freeform deformation method for representing and exploring the shape of aortic grafts. They also provide an online training and optimizing the hemodynamic surrogate model to identify the optimal design parameters. Their results achieve a 30% reduction in blood flow energy loss compared to the original coarctation by optimizing the aortic geometry.
In future studies, the researchers will improve the dimension of the design space and investigate if different assumptions result in significant differences of optimized grafts.
Published May 19, 2020