News Story
Can LPGS solid-state battery conductor degradation be mitigated?
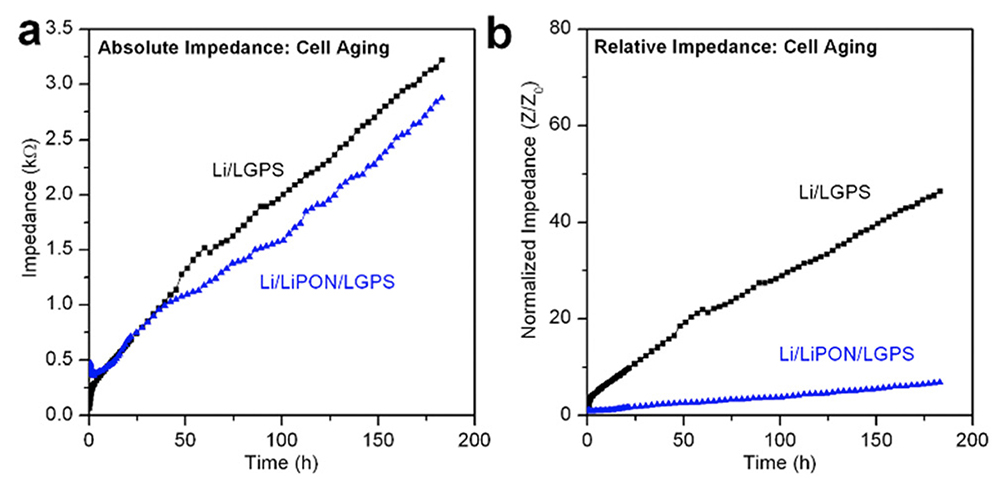
Fig. 5 from the paper. (a) Comparison of absolute cell impedance during chemical aging of bare LGPS and 20 nm LiPON-coated LGPS cells; (b) comparison of relative cell impedance during chemical aging of bare LGPS and 20 nm LiPON-coated LGPS cells. All cells were tested in a symmetric configuration with Li electrodes. [Click for larger view]
In the early 1990s, Sony commercialized the first Lithium-ion (Li-ion) batteries, and ever since, they have been the dominant power source for portable electronic devices. Today, Li-ion batteries also are powering the growing electric vehicle market. However, these batteries are the source of well-known, significant flammability concerns related to their liquid electrolytes.
To address these concerns, and to improve batteries in general, researchers are racing to develop alternatives, primarily through using solid-state electrolyte (SSE) materials.
Many SSEs have much lower ionic conductivity than the conventional liquid electrolytes used in Li-ion batteries. However, the superionic conductor SSE known as Li10GeP2S12 (LGPS) is especially promising because of its exceptionally high ionic conductivity that approximates conventional liquid electrolytes. While LGPS shows exceptional potential to fulfill the promise of solid-state batteries, unfortunately it is chemically and electrochemically unstable against both Li metal anodes and high voltage Li-ion cathode materials. This instability dramatically reduces the efficiency of LGPS-based battery systems.
A new study by University of Maryland researchers in the Sept. 15, 2022 edition of the Royal Society of Chemistry journal, Materials Advances examines ways to mitigate this electrochemical LGPS degradation.
Differentiating chemical and electrochemical degradation of lithium germanium thiophosphate and the role of atomic layer deposited protection layers was written by Professor Gary W. Rubloff (MSE/ISR) and an extensive team of his colleagues, students, alumni and postdocs.
In addition to Rubloff, the authors include Postdoctoral Researcher Yang Wang (Chem-BioChem); Rubloff’s alum Sam Klueter (MSE MS 2022); Myungsuk Lee and Junnyeong Yun of the Institute of Technology Innovation, South Korea; Rubloff’s former postdoctoral researcher Chuan-Fu Lin and Lin’s graduate student Binh Hoang (Dept. of Mechanical Engineering, Catholic University of America); graduate student Elias Kallon, co-advised by Rubloff and Assistant Professor Kevin Daniels (ECE); Cholho Lee of SK On Co., Ltd., South Korea; Rubloff’s alum and current MSE Assistant Research Scientist Alexander Kozen (MSE Ph.D. 2015); and Professor and Maryland NanoCenter Director Sang Bok Lee (Chem-BioChem/MSE).
Even though there have been studies about thermodynamic degradation in the field of Si anodes, few of these reports have investigated Li/LGPS systems or deconvolute static vs. dynamic decomposition. This study highlights the importance of deconvoluting chemical and electrochemical degradation processes, establishing motivation for further research into ASEI layers that have a high ionic conductivity and can provide both chemical as well as electrochemical stability at Li metal potentials.
The researchers applied a thin ASEI layer of ALD LiPON directly to the surface of LGPS pellets, demonstrating the effect of mitigating the interfacial degradation reactions between Li and LGPS at moderate current density electrochemical cycling. Then they qualitatively deduced the components of the degradation reactions by studying the chemical aging of the Li/LGPS interface by direct contact, and employed complementary computational studies to elucidate the effect of the ALD LiPON layer on chemical and electrochemical degradation reactions.
Their data relay fundamental insights into chemical and electrochemical degradation at the Li/LGPS interface, with significant implications on the storage, preservation, and future surface protection strategies in the practical application of the sulfide-based solid-state electrolytes.
The work shows ALD LiPON ASEI layers can partially mitigate electrochemical degradation of Li/LGPS interface during cycling. However, despite ASEI layer protection, chemical degradation still occurs in Li/LGPS cells, with 20 nm ALD LiPON unable to prevent this degradation. Still, the results clearly highlight the importance of deconvoluting chemical and electrochemical degradation processes, providing motivation for further research into ASEI layers that have a high ionic conductivity and can provide both chemical as well as electrochemical stability at Li metal potentials.
Research funding was provided by SK ON Co., Ltd., South Korea, under research agreement #19102790.
Published November 2, 2022